-
Thermal and concentration dependent energy transfer of Eu2+ in SrAl2O4
J. Bierwagen, S. Yoon, N. Gartmann, B. Walfort and H. Hagemann
Optical Materials Express, 6 (3) (2016), p793


DOI:10.1364/OME.6.000793 | unige:81858 | Abstract | Article HTML | Article PDF
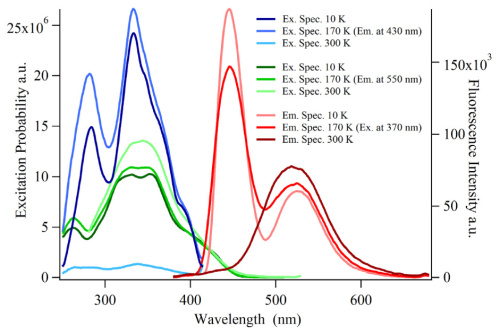
SrAl2O4 doped with europium and dysprosium is a powerful and widely used afterglow material. Within this material strontium is found in two crystallographic different sites. Due to the similar ion radii and same charge, Eu2+-ions can occupy both sites, resulting in two different Eu2+-ions, one emitting in the blue and one in the green spectral range. The blue emission is thermally quenched at room temperature. In this paper we investigate the energy transfer between different Eu ions depending on the concentration and temperature using two different approaches: lifetime measurements and integrated intensity. We find an activation energy for the thermal quenching of the blue emission of 0.195 ± 0.023 eV and a critical radius for the energy transfer of 3.0 ± 0.5 nm. This results can help in designing better afterglow materials due to the fact that with energy transfer parts of the lost emission in the blue region at room temperature can be converted to the green site.